Treated articles
- Regulations for placing treated articles on the market
- Borderline between treated articles and biocidal products
- Borderline between a treated article and a non-treated article
Regulation (EU) No. 528/2012 (BPR) applies to both biocides and treated articles.
According to Article 3 Paragraph 1 l) of the BPR, "treated articles" means any substance, mixture or article which has been treated with one or more biocidal products or which intentionally incorporate one or more biocidal products.
A treated article with a primarily biocidal function is considered a biocidal product, in accordance with Article 3 Paragraph 1 a), and must therefore be authorised as such.
Regulations for placing treated articles on the market
The placing on the market of treated articles without primary biocidal function is regulated in Article 58 of the BPR.
According to this, a treated article may only be placed on the market, when all the active substances contained in the biocidal products with which the article has been treated or which are incorporated in the article, are included in the Union list or in Annex I of the BPR for the relevant product-type and intended use, and all conditions or limitations set out in these are met.
Treated articles must be labelled by the distributor in the following cases (Article 58):
- when the manufacturer of a treated article which contains a biocidal product supplies data concerning the biocidal properties of the article, or
- when the conditions of approval of the active substance(s) involved require this.
The articles must in any case be labelled with instructions for use, including necessary safety precautions, in accordance with Article 58 Paragraph 4, when this is required for the protection of humans, animals and the environment.
According to Article 58 Paragraph 3, the labelling shall include the following information:
- a statement specifying that the treated article contains biocidal products;
- where substantiated, the biocidal property attributed to the treated article;
- the name of all active substances contained in the biocidal products;
- the names of all nanomaterials contained in the biocidal products, followed by the word "nano" in brackets;
- any relevant instructions for use, including any precautions to be taken because of the biocidal products with which a treated article was treated or which it incorporates.
The labelling shall be in the official language or languages of the Member State (unless otherwise specified) in which the treated article is to be placed on the market , and must be clearly legible and sufficiently durable.
Furthermore, the supplier of a treated article must, on application by a consumer, provide that consumer with information concerning the biocidal treatment of the treated article within 45 days, free of charge.
Transitional measures for treated articles in accordance with Article 94 of the Biocidal Products Regulation
According to the transitional regulations, treated articles could only be placed on the market independently of the active substance employed until 1 March 2017.
Since 1 March 2017, treated articles may only be placed on the market
- if the active substance employed has been approved for the relevant product-type or
- if the active substance used is listed in Annex II of the Regulation 1062/2014 for the relevant product-type and no decision has been taken not to approve the active substance, or
- if the active substance used is listed in Annex I of the Regulation 528/2012 or
- an application for approval of the active substance for the corresponding product-type was made by 1 September 2016 at the latest.
If a decision not to approve the active substance is made after 1 September 2016, then the treated articles affected may remain on the market for 180 days following this decision.
Please note: No transitional measures are envisaged for treated articles requiring labelling. These articles should accordingly have been labelled since 1 September 2013.
Borderline between treated articles and biocidal products
In order to establish whether a product is a biocidal product or a treated article, it is necessary to decide whether the product is a substance, a mixture or an article. Whilst a substance or a mixture must only demonstrate an intentional biocidal function when used in order to fulfil the definition of a biocidal product, articles are usually not biocidal products (see figure).
According to Article 3 of the REACH Regulation, an article is "an object which during production is given a special shape, surface or design which determines its function to a greater degree than does its chemical composition".
Article 3 (1) a) of the Biocidal Products Regulation defines a "Biocidal product" as a substance or mixture in the form in which it is supplied to the user. It consists of, contains or generates one or more active substances. It is intended to destroy, deter, render harmless, prevent the action of, or otherwise exert a controlling effect on, any harmful organism by any means other than mere physical or mechanical action.
According to Article 3 Paragraph. 1 l) of the Biocidal Products Regulation, “treated articles” are any substances, mixtures or articles which have been treated with one or more biocidal product or which intentionally incorporate one or more biocidal product.
A paint which contains a biocidal active substance and which is intended to protect wood from fungal infestation, would accordingly be considered a biocidal product. By contrast, a wooden fence which had been treated with wood-preserving paint to protect it from fungal infestation, would constitute a treated article. Further examples are given in the table below.
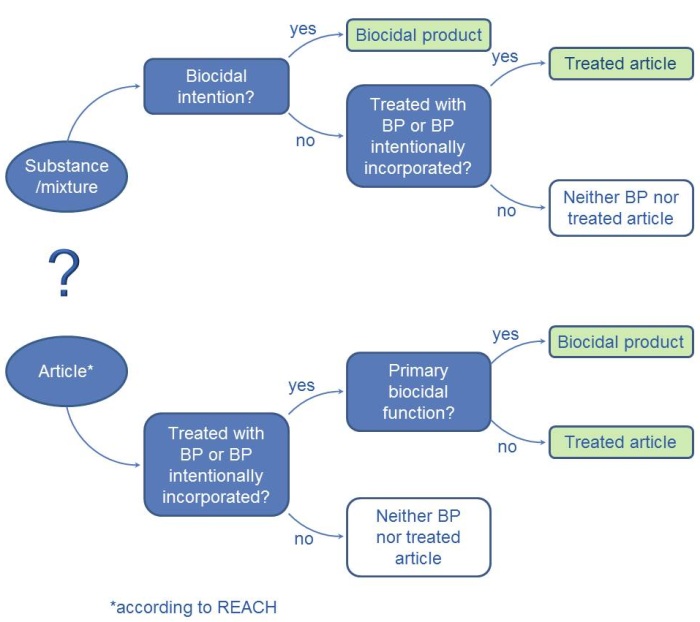
Fig.: Decision tree: Borderline between treated articles and biocidal products
Borderline between a treated article and a non-treated article
Products (both mixtures and articles) which have been treated with a biocidal product or which intentionally incorporate a biocidal product, constitute treated articles. In this respect, “intentionally incorporated” or “intentionally contain” mean that a biocidal property or function in the product is achieved by means of the treatment. Thus e.g. an in-can preservative is intentionally incorporated in a paint, in order to protect it during storage. Further examples are given in the table below.
| Biocidal product | treated article | Neither biocidal product nor treated article |
|---|---|---|
| Wood preservative (e.g. to protect against wood-destroying insects) | Garden chairs treated with this wood preservative | |
| In-can preservative (for protection from microbial damage during storage) | Paints, adhesives, sealants etc. containing an in-can preservative | Objects painted with paints containing an in-can preservative or to which adhesives or sealants containing an in-can preservative have been applied |
| Film preservatives | Paints, adhesives, sealants etc. containing a film preservative | |
| Objects painted with paints containing a film preservative | ||
| Slimicides | Paper pulps which incorporate a slimicide | Paper to which a slimicide was added during the production process, which in the finished paper no longer possess this function |
